3 Types of Gout Not 2 is my review of gout studies showing there is an extra type of gout. Because 2 types of gout causes have been recognized in the past: over-producers and under-excreters. But now we should consider a third reason for gout.
This is important as it is the foundation of all gout treatment. If you change that foundation, you create a new view of gout treatment, with new opportunities.
This is a layman’s summary of a report that changes the foundation of gout causes. It is a highly technical investigation, and I will omit much of the scientific detail from this review. The full review is widely available on the Internet, so simply search for the title.
Gout Causes Report
- Title:
- Decreased extra-renal urate excretion is a common cause of hyperuricemia
- Authors:
- Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, Yamanashi Y, Kasuga H, Nakashima H, Nakamura T, Takada Y, Kawamura Y, Inoue H, Okada C, Utsumi Y, Ikebuchi Y, Ito K, Nakamura M, Shinohara Y, Hosoyamada M, Sakurai Y, Shinomiya N, Hosoya T, Suzuki H.
- Published:
- Nat Commun. 2012 Apr 3;3:764.
This gout causes report is not presented in the usual methods, results, conclusion sequence, and I have followed the sub-headings used.
Extra-renal urate excretion is the excretion of uric acid through pathways other than the kidney – primarily the gut.
Hyperuricemia is the British English spelling of hyperuricemia, meaning high uric acid. This usually is 7mg/dL (0.42mmol/L) or higher.
Gout Causes Report Introduction
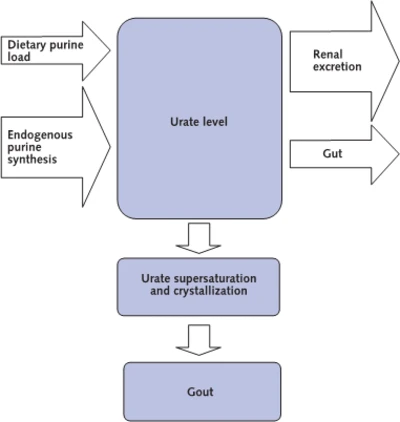
The report introduces high uric acid as the reason for gout and some kidney stones, noting that it also increases kidney and heart disease.[1,2]. It explains that humans do not have the enzyme, uricase, that other mammals degrade uric acid with.[3] Therefore we have two pathways to remove excess uric acid, with two-thirds excreted through the kidneys, leaving one-third non-kidney extraction that is mainly through the gut.[4,5] The cause of high uric acid has been described as either overproduction or underexcretion, but underexcretion has always referred to the kidneys alone.[6-9]
The kidney is the main regulator of uric acid in the blood, with the net extraction rate determined by the amount of uric acid extracted from the blood, less the amount that is returned to the blood. These processes are controlled by genes, and the report describes details of several genes involved in the control of uric acid. Gout is commonly caused by a combination of more than one genetic disorder.[10-21]
The study investigates the relationship between a genetic disorder (specifically, ABCG2 dysfunction) and uric acid excretion in humans with high uric acid and in mice.
Gout Causes Report Results
In the human analysis, 644 males were analyzed. They were grouped according to the specific genetic disorder[21] as full function, 3/4 function (mild dysfunction), 1/2 function (moderate dysfunction) and up to 1/4 function (severe dysfunction). 75.6% of these high uric acid patients had the genetic disorder.
Further analysis revealed that the disorder was associated with higher uric acid excretion through the kidneys. However, it was also associated with uric acid overproduction. The authors note a paradox in the conventional overproducer/underexcreter view, suggesting that excretion outside the kidney would account for this.
The investigators were loath to dissect their humans to investigate uric acid excretion in the gut, and so they introduced a mouse model.[22] They satisfied themselves that the profile for uric acid excretion through the kidneys matched the human model, then turned to analyze uric acid excretion through the gut. They found that the genetic disorder significantly decreased uric acid excretion through the gut. They examined excretion through the bile duct and confirmed earlier investigations that it was insignificant.[23] For patients with this genetic disorder, increased uric acid in the blood, despite higher uric acid excretion through the kidneys, is explained by decreased uric acid excretion from the gut.
Gout Causes Report Discussion
The investigation shows that this genetic disorder, previously assumed to cause overproduction of uric acid, is actually responsible for decreased non-kidney excretion.
They apply this finding to other investigations.[24-28]
In keeping with the importance of this new view of uric acid control, the authors include a lengthy interpretation of the concept noting that most overproduction explanations have been inadequate.[29] They suggest that high uric acid should now be reclassified, with overproduction split between non-kidney underexcretion and genuine overproduction.
This new concept will help pinpoint the reasons for hyperuricemia more accurately and provide a more effective therapeutic strategy for hyperuricemia and gout, leading to a good model of personalized medicine for common diseases.
Gout Causes Report Methods
The report ends with an extensive explanation of the methods used in different parts of the investigation.[30-41] The description of measuring uric acid excretion through the gut explains why the investigators were loath to test their theories on humans.
Gout Causes Report: Next Steps
What does this new classification of types of gout causes mean to us gout patients?
In the short-term, probably very little, though it might see renewed interest in Montmorillonite (Calcium Bentonite clay). I’m still waiting for some more research on Montmorillonite, but you can see some facts on my uricase page. Long-term, we should hope to see new approaches to gout control as researchers target gout caused by under-excretion of uric acid by the gut.
Leave Gout Causes Report to browse other Gout Symptoms guidelines.
Gout Causes Report References
- Feig D. I., Kang D. H. & Johnson R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359, 1811–1821 (2008). Uric Acid And Heart Risks PDF.
- Edwards N. L. The role of hyperuricemia in vascular disorders. Curr. Opin. Rheumatol. 21, 132–137 (2009).
- Yeldandi A. V. et al. . Human urate oxidase gene: cloning and partial sequence analysis reveal a stop codon within the fifth exon. Biochem. Biophys. Res. Commun. 171, 641–646 (1990).
- Sorensen L. B. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 8, 694–706 (1965).
- Sica D. A. & Schoolwerth A. in Brenner and Rector’s The Kidney (ed. B.M. Brenner) 645–649 (Saunders, 2004).
- Boss G. R. & Seegmiller J. E. Hyperuricemia and gout. Classification, complications and management. N. Engl. J. Med. 300, 1459–1468 (1979).
- Becker M. A. in The Metabolic & Molecular Bases of Inherited Disease (eds Charles R. Scriver, Barton Childs, Kenneth W. Kinzler, & Bert Vogelstein) 2513–2535 (McGraw-Hill, 2001).
- Wortmann R. L. in Harrison’s Principles of Internal Medicine (eds Anthony S. Fauci et al. .) 2444–2449 (McGraw-Hill, 2008).
- Ichida K., Hosoyamada M., Hosoya T. & Endou H. in Genetic Diseases of the Kidney (ed. Richard P. Lifton) 653–662 (Academic Press, 2009).
- Enomoto A. et al. . Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417, 447–452 (2002).
- Li S. et al. . The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 3, e194 (2007).
- Döring A. et al. . SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 40, 430–436 (2008)
- Vitart V. et al. . SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 40, 437–442 (2008).
- Anzai N. et al. . Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 283, 26834–26838 (2008).
- Matsuo H. et al. . Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 83, 744–751 (2008).
- Dehghan A. et al. . Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 372, 1953–1961 (2008).
- Kolz M. et al. . Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 5, e1000504 (2009).
- Kamatani Y. et al. . Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet. 42, 210–215 (2010).
- Cheng L. S. et al. . Genomewide scan for gout in Taiwanese aborigines reveals linkage to chromosome 4q25. Am. J. Hum. Genet. 75, 498–503 (2004).
- Woodward O. M. et al. . Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl Acad. Sci. USA 106, 10338–10342 (2009).
- Matsuo H. et al. . Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci. Transl. Med. 1, 5ra11 (2009).
- Fridovich I. The competitive inhibition of uricase by oxonate and by related derivatives of s-triazines. J. Biol. Chem. 240, 2491–2494 (1965).
- Sorensen L. B. & Levinson D. J. Origin and extrarenal elimination of uric acid in man. Nephron 14, 7–20 (1975).
- Shimizu T. et al. . PDZK1 regulates breast cancer resistance protein in small intestine. Drug Metab. Dispos. 39, 2148–2154 (2011).
- Anzai N. et al. . The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J. Biol. Chem. 279, 45942–45950 (2004).
- Huls M. et al. . The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 73, 220–225 (2008).
- Maliepaard M. et al. . Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61, 3458–3464 (2001).
- Doyle L. A. & Ross D. D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 22, 7340–7358 (2003).
- Zaka R. & Williams C. J. New developments in the epidemiology and genetics of gout. Curr. Rheumatol. Rep. 8, 215–223 (2006).
- Wallace S. L. et al. . Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 20, 895–900 (1977).
- Puig J. G. & Mateos F. A. Clinical and biochemical aspects of uric acid overproduction. Pharm. World Sci. 16, 40–54 (1994).
- Perez-Ruiz F., Calabozo M., Erauskin G. G., Ruibal A. & Herrero-Beites A. M. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum. 47, 610–613 (2002).
- Wortmann R. L. Gout and hyperuricemia. Curr. Opin. Rheumatol. 14, 281–286 (2002).
- Urano W. et al. . Sodium-dependent phosphate cotransporter type 1 sequence polymorphisms in male patients with gout. Ann. Rheum. Dis. 69, 1232–1234 (2010).
- The guideline revising committee of Japanese Society of Gout and Nucleic Acid Metabolism in Guideline for the Management of Hyperuricemia and Gout (ed. The guideline revising committee of Japanese Society of Gout and Nucleic Acid Metabolism) 60–72 (Medical Review, 2010).
- Ichida K. et al. . Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria. J. Clin. Invest. 99, 2391–2397 (1997).
- Daimon M. et al. . Large-scale search of SNPs for type 2 DM susceptibility genes in a Japanese population. Biochem. Biophys. Res. Commun. 302, 751–758 (2003).
- Hayashi H., Takada T., Suzuki H., Akita H. & Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology 41, 916–924 (2005).
- Takada T., Weiss H. M., Kretz O., Gross G. & Sugiyama Y. Hepatic transport of PKI166, an epidermal growth factor receptor kinase inhibitor of the pyrrolo-pyrimidine class, and its main metabolite, ACU154. Drug Metab. Dispos. 32, 1272–1278 (2004).
- Hall I. H., Scoville J. P., Reynolds D. J., Simlot R. & Duncan P. Substituted cyclic imides as potential anti-gout agents. Life Sci. 46, 1923–1927 (1990).
- Kitamura Y. et al. . Determination of probability distribution of diplotype configuration (diplotype distribution) for each subject from genotypic data using the EM algorithm. Ann. Hum. Genet. 66, 183–193 (2002).
3 Gout Types Document Change History
 To read the document change history, click the GoutPal History image on the right.
To read the document change history, click the GoutPal History image on the right.
Do you have suggestions for improving 3 Gout Types? Then, please add your idea to GoutPal’s Suggestion Box. Or, send the Feedback Form, below.
Please give your feedback
Did this page help you? If yes, please consider a small donation. Your donations help keep GoutPal's gout support services free for everyone.
If not, please tell me how I can improve it to help you more.
- YouTube
- The gout forums.